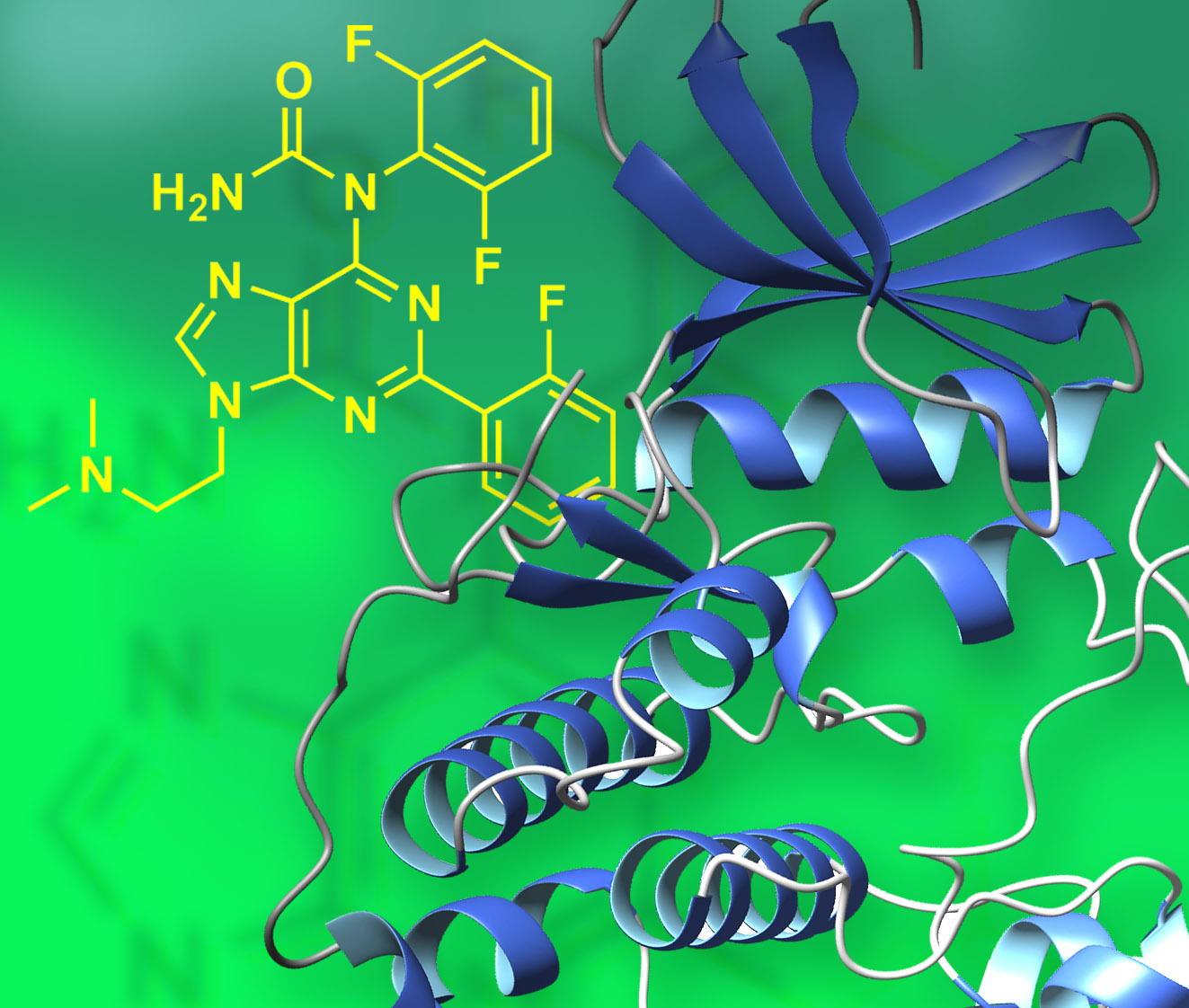
Arylated purines exhibit modified sterics, conformational preferences, H-bonding and hydrophobic interactions with nucleic acid and protein targets. Metal-mediated C-C and C-N coupling with arylboronic acids introduces increased structural diversity leading to novel probes and drugs, as illustrated by targeting specific protein kinases. See Strouse, Jeselnik, and Arterburn, Acta Chim. Slov. 2005, 52(3), 187-199.